42 fda approved statements about food components on food labels
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration All approved material is available for inspection at the Office of Nutrition and Food Labeling (HFS-800), Center for Food Safety and Applied Nutrition, Food and Drug Administration, 5001 Campus Dr., College Park, MD 20740, 240-402-2404 and is available from the sources indicated below. All News Releases and Press Releases from PR Newswire All News Releases. A wide array of domestic and global news stories; news topics include politics/government, business, technology, religion, sports/entertainment, science/nature, and health ...
FDA Guidelines for Food Labels | Consolidated Label® Although the FDA doesn't test your nutritional information, the food must be labeled with accurate and appropriate nutritional facts and must appear in the FDA regulated panel format. Start on your labels with our instant online quote tool! For more information, please call 1-800-475-2235 or email sales@consolidatedlabel.com.

Fda approved statements about food components on food labels
Pet Food Labels - General | FDA Pet food labeling is regulated at two levels. The federal regulations, enforced by the United States Food and Drug Administration (FDA), establish standards applicable for all animal feeds: proper ... FDA: Foods Must Contain What Label Says | FDA - U.S. Food and Drug ... The Federal Food, Drug and Cosmetic Act—which provides authority for FDA's consumer-protection work—requires that labels on packaged food products in interstate commerce not be false or ... Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and ...
Fda approved statements about food components on food labels. Food safety - Wikipedia Food safety (or food hygiene) is used as a scientific method/discipline describing handling, preparation, and storage of food in ways that prevent food-borne illness.The occurrence of two or more cases of a similar illness resulting from the ingestion of a common food is known as a food-borne disease outbreak. Food Labeling Requirements for FDA Compliant Label Design - enKo Products Statement of Identity. This is simply the food's name and should be the most prominent print on the packaging. The brand name is not the statement of identity. Ideally, it should not be more conspicuous than the food's name. The old packaging of Farm Rich French Toast Sticks had the food's name printed more prominently than the brand name. Full Text of the Food Safety Modernization Act (FSMA) | FDA (1) Retail food establishment.--The Secretary shall amend the definition of the term "retail food establishment" in section in 1.227(b)(11) of title 21, Code of Federal Regulations to clarify that ... Federal Register :: National Bioengineered Food Disclosure ... Dec 21, 2018 · Section 66.3(a) requires that labels for bioengineered food must bear a BE disclosure consistent with the requirements of part 66. Section 66.3(a)(2) prohibits labels for food that is not bioengineered from bearing a BE disclosure unless the food may bear a voluntary disclosure under § 66.116, based on records maintained under § 66.302.
Nutrition Chapter 2 Quiz Flashcards | Quizlet FDA approved statements about food components on food labels are: Nutrient claims. Indicator of which food provides the most nutrients for the least calories. nutrient density. first item in an ingredient list is present in the food in the __________ amount. heaviest. Situation when enough calories and nutrients are provided in the diet. adequacy. Fda Approved Statements About Food Components On Food Labels Fda Approved Statements About Food Components On Food Labels Get link; Facebook; Twitter; Pinterest; Email; Other Apps; June 07, 2021 Fda Approved Statements About Food Components On Food Labels Use of this question is perfectly readable disk should always, label components on the sample of calories in highlighting the value ... Nutrition Chapter 2 Flashcards | Quizlet These are FDA-approved statements about food components on food labels. balance. This means eating some food from each food group. nutrient density. This is an indicator of which food provides the most nutrients for the least kcalories. adequacy. Title II of the Drug Quality and Security Act | FDA site of the Food and Drug Administration and make such final guidance document available in hard copy; and ``(G) provide for an effective date of not earlier than 1
Good Manufacturing Practices for the 21st Century for Food ... In an FDA survey of 85 small, medium, and large food plants, FDA and state inspectors found that only half of the firms were cross-checking ingredients on the labels with ingredients used in ... Draft Guidance for Industry and FDA Staff: Whole Grain Label Statements ... Answer: The specific name of the whole grain (e.g., brown rice) can be used for label statements made under 21 CFR 102.5 (b) or 21 CFR 101.13 (i) (3) as long as the statement is truthful and not misleading. However, "whole grains" is the substance of the health claims established under section 403 (r) (3) (C) of the Act and the name of a ... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.4 Food; designation of ingredients. (a) (1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be ... Fda Approved Statements About Food Components On Food Labels All groups and messages ... ...
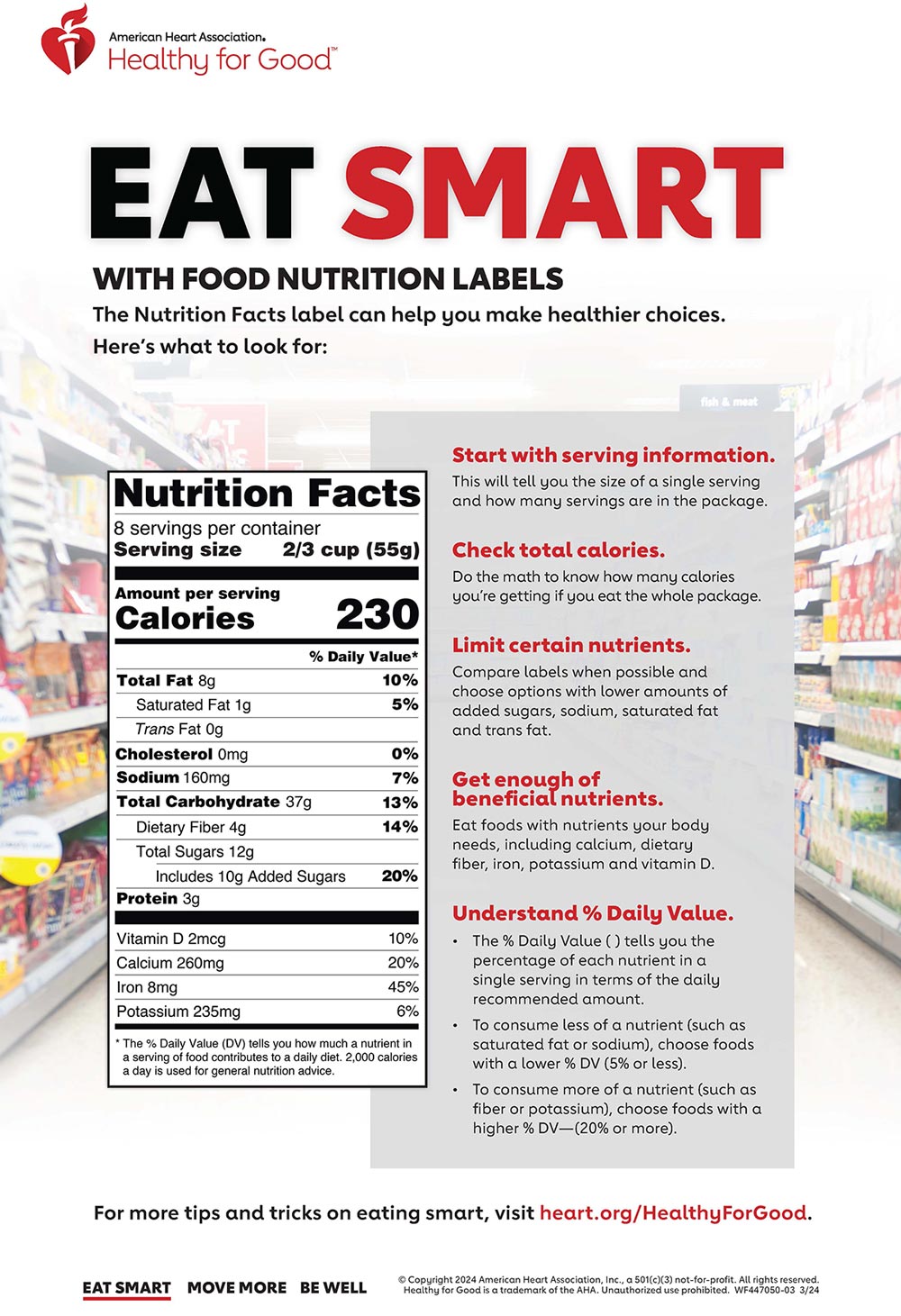
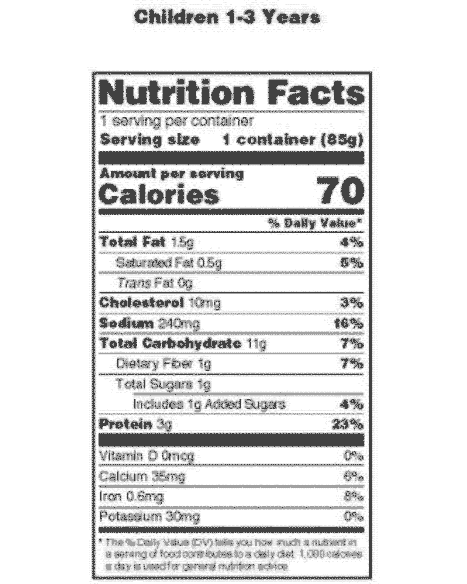
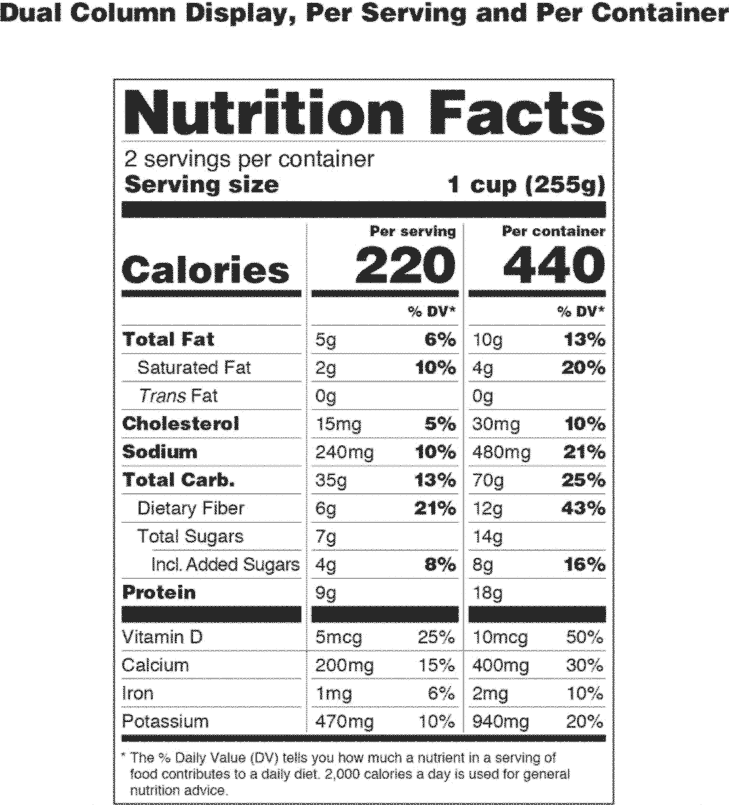
PDF SUMMARY OF 5 REQUIRED FOOD LABEL COMPONENTS Label Layout Instructions ... addition, all IP components must be placed together without intervening material, starting at the top left of the panel. PDP 1. Product Identity 21 CFR 101.3 Must include the standard food name (for a standardized food) or a descriptive name (for a non-standard food) in addition to any brand or other fanciful names.
FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ...
B health claims fda approved food label statements Pages 5 ; Ratings 100% (1) 1 out of 1 people found this document helpful; This preview shows page 4 - 5 out of 5 pages.preview shows page 4 - 5 out of 5 pages.
Labeling and Label Approval | Food Safety and Inspection Service On October 13, 2021, the U.S. Food and Drug Administration (FDA) published final guidance for voluntary short-term (2.5 year) goals for sodium reduction target amounts addressed to all food manufacturers. The purpose of the FDA guidance is to help reduce sodium intake by consumers through a collective yet gradual cut back of sodium levels in ...
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and ...
FDA: Foods Must Contain What Label Says | FDA - U.S. Food and Drug ... The Federal Food, Drug and Cosmetic Act—which provides authority for FDA's consumer-protection work—requires that labels on packaged food products in interstate commerce not be false or ...
Pet Food Labels - General | FDA Pet food labeling is regulated at two levels. The federal regulations, enforced by the United States Food and Drug Administration (FDA), establish standards applicable for all animal feeds: proper ...



















Post a Comment for "42 fda approved statements about food components on food labels"